U.S. Oncology Genomics Tracker
The focus of the U.S. Oncology Genomics Tracker is to characterize the current clinical use of genomic testing across clinical applications (e.g., genomic profiling, MRD / recurrence monitoring, treatment response monitoring) and track how use changes over time. Respondents were asked about use of genomic testing within routine clinical practice within the past 3 months.
The survey was distributed randomly to U.S. oncologists. Respondents were selected to complete the survey if they were an actively-practicing board-certified medical oncologist or hematologist-oncologist, U.S.-based and had seen at least 40 patients over the past 3 months. Respondents were not screened out of the survey if they were not currently using, or had not previously used, genomics for biomarker testing.
Survey Table of Contents:
Introduction and Methodology
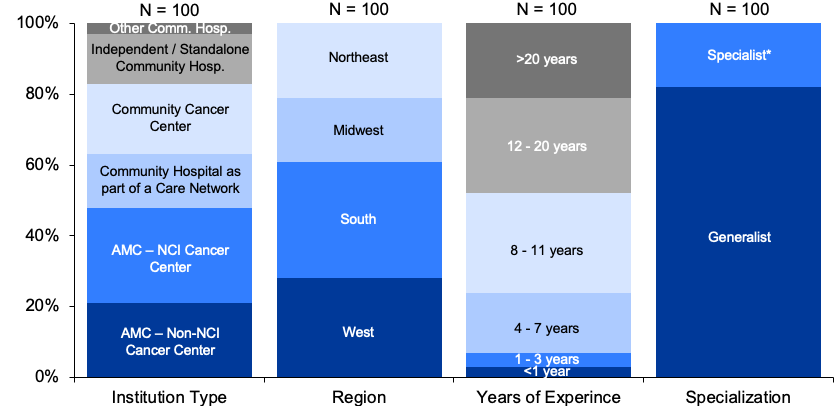
- Survey Demographics
- Survey Scope and Definitions
- Survey Limitations / Considerations
Survey Findings
- Executive Summary, Genomic Profiling & MRD / Recurrence Monitoring
- Executive Summary, Treatment Response Monitoring & Commercial Activity
Survey Findings – Genomic Profiling
- In the past 3 months, what share of your newly-diagnosed patients, received an NGS-based genomic profiling test for Tx selection? (Overall)
- In the past 3 months, what share of your newly-diagnosed patients received an NGS-based genomic profiling test for Tx selection? (AMC vs. CH)
- Of those that received NGS-based genomic profiling testing in the last 3 months, what share was tissue-based vs. blood (ctDNA)-based?
- Of those that received NGS-based GP* testing in the last 3 months, what share was small panels vs. large panels vs. WES / WGS?
- What is your level of awareness / familiarity with each of the following genomic profiling providers (tissue and / or blood-based)? (N=100)
- Which companies do you perceive to have the strongest offerings in genomic profiling (including tissue and/or liquid)?
- (1) What share of your tissue-based genomic profiling in the past 3 mo. was performed using each of the following providers? (2) In the next 12 mo. how do you expect your use of providers (by share) to change?
- For your top tissue-based genomic profiling vendor, what are the top 3 reasons why you order from this provider?
- What are your top pain points as it relates to comprehensive genomic profiling among tissue providers?
- (1) What share of your blood-based (ctDNA) genomic profiling in the past 3 months was performed using each of the following providers? (2) Over the next 12 months, how do you expect your use of providers (by share) to change?
- For your top blood-based genomic profiling vendor, what are the top 3 reasons why you order from this provider?
- What are your top pain points as it relates to comprehensive genomic profiling among blood-based providers?
- Considering only the past 3 months, please rank up to 4 of the top genomic profiling companies by number of sales representative interactions
- In the past 3 mo., how many times have you been contacted by sales reps about genomic profiling offerings from your top 4 companies?
- Rank up to 4 companies that, in the past 3 mo., had the most noticeable / memorable news, announcements, pubs*, etc. in genomic profiling
Survey Findings – MRD / Recurrence Monitoring
- How would you rate your familiarity with ctDNA-based MRD and monitoring testing in solid tumors? (N = 100)
- In the last 3 months, for patients receiving neoadjuvant treatment, what share received a blood-based (ctDNA) response monitoring test?
- In the last 3 months, for patients within 6 months of completion of curative intent treatment, what share received a ctDNA MRD test?
- In the last 3 months, for patients 6+ month from completion of curative intent treatment, what share received a ctDNA recurrence monitoring test?
- What is your level of confidence in the clinical utility / performance of MRD / monitoring testing?
- What is your level of awareness / familiarity with the solid tumor MRD / recurrence monitoring offerings from each of the following providers?
- (1) In the past 3 mo., what share of MRD and monitoring* testing was ordered from each of the following providers? (2) Over the next 12 months, how do you expect your use of providers (by share) will change?
- For your top MRD / monitoring testing vendor, what are the top 3 reasons why you order from this provider?
- Which companies do you perceive to have the strongest offerings in MRD / Recurrence Monitoring?
Survey Findings – Treatment Response Monitoring
- How would you rate your familiarity with ctDNA-based treatment response monitoring testing in solid tumors?
- In the last 3 month, for your adjuvant stage patients within 6 mo. of 1L systemic Tx, what share received a ctDNA Tx resp. monitoring test?
- Of those that receive Tx response monitoring testing, how many tests do patients typically get over the course of their treatment journey?
- What is your level of confidence in the clinical utility / performance of treatment response monitoring testing?
- What is your level of awareness / familiarity with each of the following treatment response monitoring offering providers?
- (1) In the past 3 mo., what share of Treatment Response Monitoring testing was ordered from each of the following providers? (2) Over the next 12 months, how do you expect your use of providers (by share) will change?
- For your primary Treatment Response Monitoring vendor, what are the top 3 reasons why you order from this provider?
- Which companies do you perceive to have the strongest offerings in Treatment Response Monitoring?
- Considering the past 3 months, rank up to 4 of the top MRD / Treatment Response Monitoring companies by number of sales rep. interactions
- In the past 3 mo., how many times have you been contacted by sales reps about MRD or TRM* offerings from your top 4 companies?
- What are the top 4 companies you noticed the most memorable news, announcements, publications, etc. in MRD / Tx Response Monitoring?
Appendix – Intro to DeciBio
Purchase Options
Experience the DeciBio Difference
Experience the DeciBio Difference
- $ 50,000.00 USD




